1. Article overview
The lattice strain caused by the soft perovskite lattice and the crystal distortions such as lattice surface and grain boundary defects, to a large extent, determine the charge extraction-transfer kinetics and recombination, leading to perovskite solar cells (perovskite solar cells). Battery) is less efficient. Here, the author proposes a strategy to significantly reduce the tensile strain of the surface lattice by adding inorganic two-dimensional Cl-end Ti3C2 (Ti3C2Clx) MXene to the volume and surface of the CsPbBr3 film. Due to the strong interaction between the Cl atom in Ti3C2Clx and the uncoordinated Pb2+ in the CsPbbr3 lattice, the expanded perovskite lattice is compressed and restricted as a lattice "band", in which the Pb-Cl bond forms the "glue" Function, 2DTi3C2 fixes the crystal lattice. Finally, on the best all-inorganic Cspbbr3PSC, at an ultra-high open circuit voltage as high as 1.702V, the champion efficiency reached 11.08%, which is the highest efficiency record for this type of perovskite solar cell so far. In addition, the performance of the unencapsulated device at a relative humidity of 80%, 100 days and 85°C has little change.
2. Graphic guide
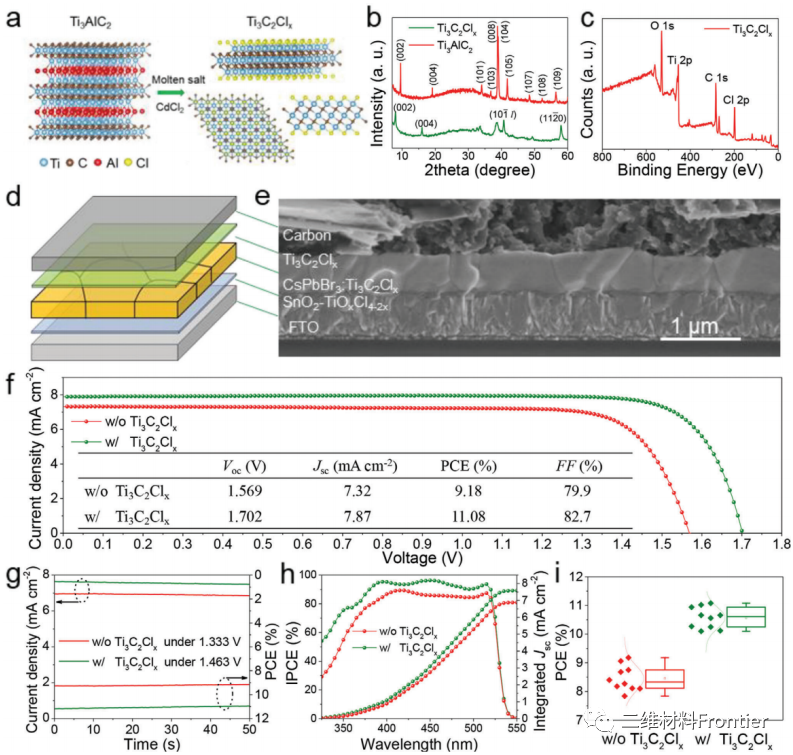
Figure 1.a) Schematic diagram of preparing Ti3C2ClxMXene in cadmium chloride molten salt using Ti3AlC2MAX. b) XRD mode of Ti3AlC2MAX and Ti3C2ClxMXene. c) XPS spectrum of Ti3c2ClxMXene. d) Architecture and e) Cross-sectional scanning electron microscope image of all inorganic PSC. f) The J-V curve of the control and optimization equipment under reverse scanning, g) the steady-state power output, and h) the IPCE spectrum of the perovskite solar cell. i) The statistical PCE distribution of the original perovskite solar cell and the optimal perovskite solar cell.
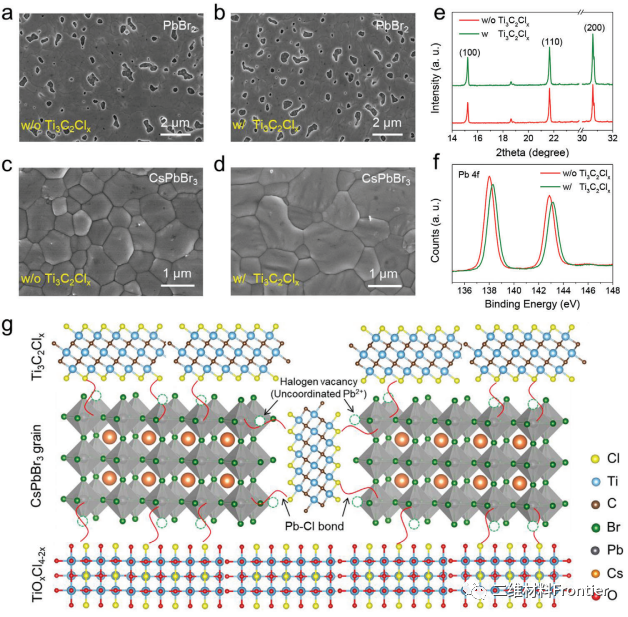
Figure 2. PbBr2 film and CsPbBr3 perovskite film a, c, b, d, Ti3C2Clx additives. e) XRD mode and XPS spectrum of Pb4f in CsPbBr3 perovskite film. g) Schematic diagram of full defect passivation of CsPbBr3 film by Ti3C2ClxMXene.
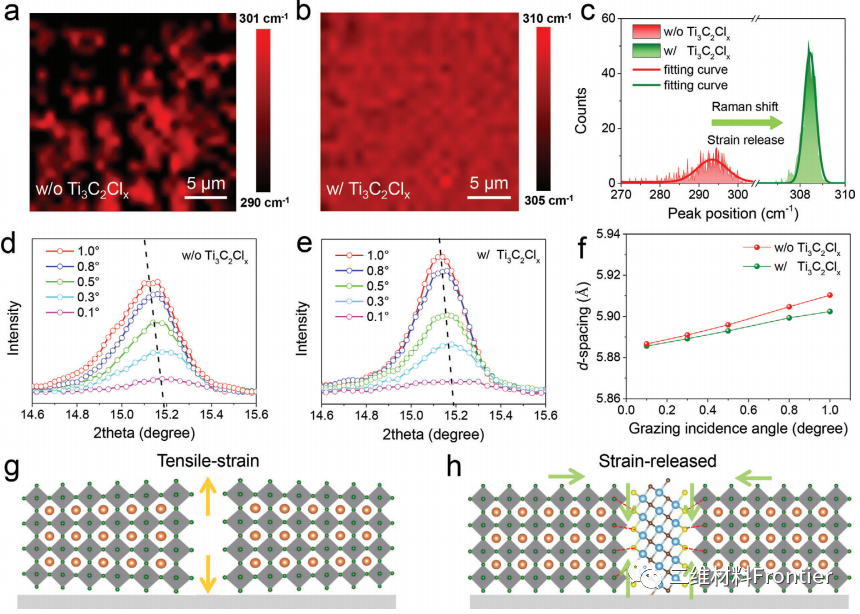
Figure 3. The Raman mapping images of the CsPbBr3 perovskite film a) without and b) containing Ti3C2ClxMXene, c) the Raman peak distribution statistics data corresponding to different perovskite films. Ti3c2Clx modified perovskite film d) and e) CsPbBr3 (100) plane GIXRD pattern. f) The d-spacing value obtained from the GIXRD mode as a function of the angle of incidence. g) Schematic diagram of residual strain in original CsPbBr3 grains, h) Schematic diagram of release strain in CsPbBr3 grains reinforced with Ti3C2ClxMXene.
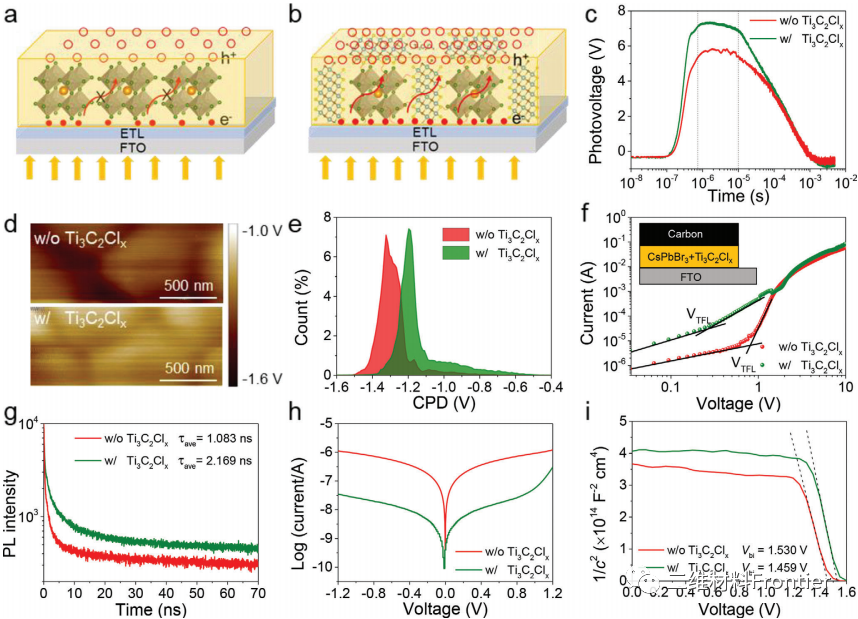
Figure 4. Schematic diagram of photogenerated carrier transfer in a) original film and b) perovskite-containing ti3c2Clx film. c) TSPV curves of different perovskite solar cells. d) KPFM image and e) the corresponding CPD distribution of the perovskite film. f) Dark J-V curves of hole-only devices with and without Ti3C2ClxMXenn. g) TRPL curves of perovskite films with and without ti3c2Clx. h) Dark J-V curve, i) Mott-Schottky curve of different perovskite solar cells.
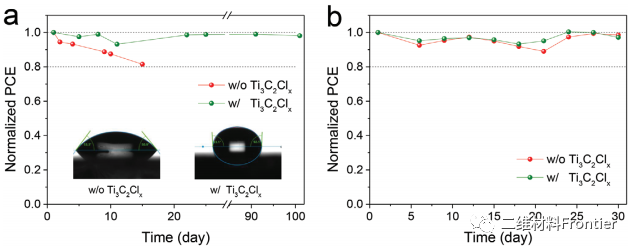
Figure 5. The long-term stability of unpackaged devices with and without Ti3C2Clx in the air under the conditions of a) 25°C, 80%RH and b) 85°C, 40%RH.
3. Full text summary
In summary, we propose a new defect passivation and strain release strategy, adding the cl-side Ti3C2MXene to the volume and surface of the perovskite film to achieve a stable and efficient all-inorganic CsPbBr3 perovskite solar cell. Due to the formation of the interface between Pb2+ ions in the perovskite lattice and the terminating Cl atom in Ti3C2ClxMXCene, the defects and expansion lattices at the interface and grain boundaries of the perovskite film are obviously healed and play the role of the surface lattice "band", where Pb- The Cl bond stands for "glue", and Ti3C2 fixes the crystal lattice. The results show that the efficiency of the best all-inorganic CsPbBr3PSC is as high as 11.08%, and the ultra-high Voc is 1.702V, which is the highest PCE and voltage record of the CsPbBr3 solar cell. In addition, unencapsulated solar cells have good stability under high humidity (80%RH) and high temperature (85°C) conditions, respectively, within 100 days and 30 days. Our research results have opened up a new way to prepare high-quality defect-free perovskite films, which is not only conducive to the preparation of high-performance perovskite solar cells, but also to other perovskite-based optoelectronic devices.
Article link:
https://doi.org/10.1002/advs.202101418
This information is sourced from the Internet for academic exchanges only. If there is any infringement, please contact us to delete it immediately.